Showing 41–60 of 1565 resultsSorted by latest
Browse By Categories
By Price
By Category
- A
- Abdos
- B
- Body Care
- C
- Category
- Cold & Flu
- D
- Dosimetry
- E
- Elisa
- F
- G
- Glassco
- ANALYTICAL LABORATORY BENCHTOP INSTRUMENTS
- LAB ESSENTIALS
- LABORATORY GLASSWARE
- Adapter
- Automatic Burette
- Beaker
- Burette
- Columns
- Condensers
- Conical Flask
- Crucibles
- Cylinders
- Dessicators
- Dissolution Vessel
- Distillation
- Extraction Apparatus
- Filtration Assembly
- Funnels
- Joints
- Pasteur Pipette
- Petri Dishes
- Pipettes
- Reagent Bottles
- Stopcocks
- Stoppers
- Test Tubes
- Tube centrifuge
- Utility Sets
- Vials
- Volumetric Flask
- LIQUID HANDLING
- METALWARE
- PLASTICWARE
- RUBBERWARE
- WATER DISTILLATION
- H
- Health & House Hold
- Healthy Products
- House Hold & Protection
- I
- J
- JuniperLifeSciences
- K
- L
- Laboratory Chemicals
- Laboratory Cosumables
- Laboratory Equipments
- Laboratory Plasticwares
- Laboratory Services
- M
- Medicines & Treatments
- Microexpress
- Molychem
- MP Biologicals
- APPLICATIONS
- Diagnostics
- Life Sciences
- Animal Diets and Feeds
- Antibodies
- Biochemicals
- Cell Biology
- Chemicals
- Electrophoresis
- General Lab Equipment and Reagents
- Molecular Biology
- Sample Preparation
- N
- No
- O
- P
- Protection Virus
- R
- S
- T
- Tag
- Takara
- Antibodies and ELISA
- Automation systems
- cDNA synthesis
- cDNA synthesis accessories
- cDNA synthesis kits
- Purified cDNA & genomic DNA
- Purified total RNA and mRNA
- RACE kits
- Reverse transcriptases
- Cell biology assays
- Cloning
- Agarose gel electrophoresis
- and cloning vectors
- Cloning vectors
- Chloramphenicol-resistant pUC vectors
- DNA cloning vectors
- E. coli-yeast shuttle vectors
- Kanamycin resistant pUC vectors
- Lambda DNA
- M13mp18 RF phage vector
- M13mp18 virion DNA
- pBR322 DNA vector
- PhiX174 RF I DNA
- Plant transformation vectors
- pPTR shuttle vectors
- pUC118/119 vectors
- pUC18 and pUC19 vectors
- T-Vectors pMD20 and pMD19
- Thermus thermophilus HB8 genomic DNA
- Linkers and primers
- Cloning vectors
- Competent cells
- In-Fusion Cloning
- Ligation enzymes
- Ligation kits
- Linkers
- Cloning vectors
- Chloramphenicol-resistant pUC vectors
- DNA cloning vectors
- E. coli-yeast shuttle vectors
- Kanamycin resistant pUC vectors
- Lambda DNA
- M13mp18 RF phage vector
- M13mp18 virion DNA
- pBR322 DNA vector
- PhiX174 RF I DNA
- Plant transformation vectors
- pPTR shuttle vectors
- pUC118/119 vectors
- pUC18 and pUC19 vectors
- T-Vectors pMD20 and pMD19
- Thermus thermophilus HB8 genomic DNA
- Linkers and primers
- Cloning vectors
- Modifying enzymes
- Mutagenesis kits
- Nucleic acid extraction
- primers
- Cloning vectors
- Chloramphenicol-resistant pUC vectors
- DNA cloning vectors
- E. coli-yeast shuttle vectors
- Kanamycin resistant pUC vectors
- Lambda DNA
- M13mp18 RF phage vector
- M13mp18 virion DNA
- pBR322 DNA vector
- PhiX174 RF I DNA
- Plant transformation vectors
- pPTR shuttle vectors
- pUC118/119 vectors
- pUC18 and pUC19 vectors
- T-Vectors pMD20 and pMD19
- Thermus thermophilus HB8 genomic DNA
- Linkers and primers
- Cloning vectors
- Restriction enzymes
- X-Gal and IPTG
- COVID-19 research
- Gene function
- Fluorescent proteins
- Gene editing
- Cre recombinase
- CRISPR-Cas9
- AAV systems
- Cas9 antibodies
- Cas9-sgRNA gesicle production
- Genome-wide sgRNA library system
- Genotype confirmation kit
- GMP recombinant Cas9
- In vitro transcription and screening kits
- Indel identification kit
- Knockin screening kit
- Lentiviral systems
- Long ssDNA for knockins
- Mutation detection kits
- Plasmid systems
- Recombinant Cas9 protein
- RNA transfection
- SNP screening
- iDimerize inducible protein interaction systems
- Mammalian expression plasmids
- ProteoTuner protein control systems
- T-cell transduction and culture
- Tet-inducible expression systems
- Tet systems legacy products
- Tet-approved FBS
- Tet-inducible miRNA systems
- Tet-On 3G systems
- Adeno-X Tet-On 3G inducible expression system
- Tet-On 3G cell lines
- Tet-On 3G systems
- Tet-On 3G systems?bicistronic
- Tet-On 3G systems?bidirectional
- Tet-On 3G systems?EF1-alpha promoter
- Tet-On 3G systemsbicistronic
- Tet-On 3G systemsbidirectional
- Tet-On 3G systemsEF1-alpha promoter
- Tet-On 3G?lentiviral
- Tet-On 3G?retroviral
- Tet-On 3Glentiviral
- Tet-On 3Gretroviral
- Tet-One systems
- TetR monoclonal antibody
- Transfection reagents
- Viral transduction
- Adeno-associated virus (AAV)
- Adenovirus
- Lentivirus
- Retrovirus
- Next-generation sequencing
- DNA-seq
- Epigenetics and small RNA sequencing
- Immune profiling
- NGS accessories
- RNA-seq
- Whole genome amplification
- Nucleic acid purification
- Accessories and components
- DNA cleanup kits
- Genomic DNA purification kits
- Plasmid purification kits
- RNA cleanup kits
- RNA purification kits
- Viral DNA and RNA purification kits
- PCR
- Application-specific PCR
- Custom business friendly and automation-ready solutions
- Direct PCR
- Fast PCR
- GC rich PCR
- High-fidelity PCR
- High-yield PCR
- Long-range PCR
- Molecular diagnostic products
- Other PCR-related products
- PCR master mixes
- PCR thermal cyclers
- Standard PCR
- Protein research
- Accessory enzymes
- Antibodies and immunoprecipitation
- Expression vectors & systems
- Glycobiology
- Mass spectrometry reagents
- Protein sequencing
- Purification products
- SDS-PAGE & western blotting
- Two-hybrid and one-hybrid systems
- Real-time PCR
- Application-specific qPCR
- Environmental
- Food
- Bacterial quantification in food
- Campylobacter detection
- Enterobacteriaceae detection
- Escherichia coli O-157:H7 detection 2.0
- Escherichia coli O157:H7 and O26 detection
- Escherichia coli O157:H7 detection
- Listeria monocytogenes detection
- Norovirus detection
- Salmonella detection
- Staphylococcus aureus detection
- Vibrio (tdh gene) detection
- Human disease
- Mycoplasma detection
- Real-time PCR kits
- Real-time PCR primer sets
- References and standards for qPCR
- Reverse transcription prior to qPCR
- RNA extraction and analysis for real-time qPCR
- Application-specific qPCR
- Stem cell research
- Accessories
- Human iPS cell gene editing systems
- Media and supplements
- Stem cells and stem cell-derived cells
- Human induced pluripotent stem cells
- Human multipotent mesenchymal progenitor cells
- Human neural stem cells
- Human stem cell-derived beta cells
- Human stem cell-derived cardiomyocytes
- Human stem cell-derived definitive endoderm cells
- Human stem cell-derived hepatocytes
- Human stem cell-derived intestinal epithelial cells
- MiraCell cardiomyocytes
- MiraCell endothelial cells
- U
- V
- W
- X
- Y
-
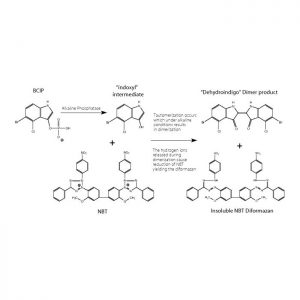
BCIP/NBT, liquid substrate, 100 mL
(5-Bromo-4-chloro-3-indolyl Phosphate/Nitroblue Tetrazolium)Stabilized Chromogen SolutionSpecially prepared for immunoblotting procedures only. Concentration: 0.577 mmol/liter BCIP; 0.122 mmol/liter NBT.
-
BCIP/NBT, liquid substrate plus, 100 mL
BCIP/NBT Liquid Substrate Plus is a stabilized chromogen solution. It has a greater sensitivity and produces little or no background staining compared with other alternatives. It is ready to use solution.
-
3,3-Diaminobenzidine tablets, 100 tabs
Used in immunohistology and immunoblotting as a precipitating substrate for the localization of peroxidase activity. Insoluble end product is resistant to alcohol and fading, making it ideal for immunohistochemistry.
-
BCIP/NBT, stable alkaline phosphatase substrate, 100 mL
Stabilized Chromogen Solution
This alkaline phosphatase chromogen solution contains the two components in a stabilized solution which may be used as the final step in color development. The resulting product is a permanent, deep-purple stain. It enhances results in both ELISA and immunohistology assays utilizing peroxidase and alkaline phosphatase. -
TMB (3,3′,5,5′ Tetramethylbenzidine)
3,3′,5,5′-Tetramethylbenzidine (TMB) is a noncarcinogenic substitute (Ames test negative) for benzidine suitable as peroxidase substrate for use in ELISA procedures. The substrate produces a soluble end product that is pale blue in color and can be read spectrophotometrically at 370 or 620-650 nm. The TMB reaction may be stopped with 2 M H2SO4 (resulting in a yellow color), and read at 450 nm. A sensitive and specific reagent for the detection of blood, assay of hemoglobin, assay of peroxidases.
-
Ovalbumin, 5x crystallized, 5 g
Prepared from chicken egg white by Ion exchange.
-
Albumin, bovine, Cohn fraction V, > 96%
Albumins are a group of acidic proteins which occur plentifully in the body fluids and tissues of mammals and in some plant seeds.
-
Albumin, rat, fraction V, 100 mg
Lyophilized
This albumin is prepared from rat serum. -
Transferrin, human, iron saturated, 1 g
Transferrin is a glycoprotein with homologous N-terminal and C-terminal iron-binding domains.
-
Transferrin, human, iron poor (apo), 1 g
Transferrin is a glycoprotein with homologous N-terminal and C-terminal iron-binding domains.
-
Albumin, Human, fraction V (fatty acid free), 5 g
Tested negative for the presence of Hepatitis B Surface Antigen (HBsAg), HIV I/II, HIV-1Ag, HCV, ALT and Syphilis
-
Albumin, human, 30% solution, 50 mL
Tested negative for the presence of Hepatitis B Surface Antigen (HBsAg), HIV I/II, HIV-1Ag, HCV, ALT and Syphilis
-
Albumin, human crystallized (1x Cohn crystallized)
ALBUMIN, HUMAN is recommended for use in absorption, distribution, metabolism, and excretion (ADME) pharmacological research; cell culture; drug delivery research; and cryopreservation of cells. Prepared using Cohn method IV. The material is non-reactive for HbsAg, Anti-HCV 3.0, Anti-HIV-1/2 and STS (RPR). This material is also negative for HIV-1 and HCV by PCR. Product Description Albumins are a group of simple proteins found in the body fluids and tissues of animals, and in some plant seeds. It is a single polypeptide chain with one free sulfhydryl group (Cys34) and 17 intrachain disulfide bonds. Biochem/physiol Actions Albumins are soluble monomeric proteins found in the body fluids and tissues of animals and in some plant seeds. Serum albumin functions as a carrier protein for steroids, fatty acids, and thyroid hormones. Serum albumins are also vital in regulating the colloidal osmotic pressures of blood.
-
Albumin, human, fraction V, 10 g
This human protein is prepared by a modification of the Cohn procedure. Albumin may be used to eliminate background interference in ELISA’s or other enzyme assay systems. It is also used in absorption, distribution, metabolism, and excretion (ADME) pharmacological research; cell culture; drug delivery research; and cryopreservation of cells. Human and bovine albumins contain 16% nitrogen and are often used as standards in protein calibration studies. Due to their free hydrophobic region fatty acid free albumins are used to solubilize lipids in tissue culture, and are also used as blocking agents in Western blots or ELISA applications. Globulin free albumins are suitable for use in applications where no other proteins should be present (e.g., electrophoresis). Serum albumin functions as a carrier protein for steroids, fatty acids, and thyroid hormones, and is vital in regulating the colloidal osmotic pressures of blood. Albumin is also seen to bind to exogenous substances, particularly drugs (e.g., ibuprofen, warfarin), and strongly influence their pharmacokinetics. Oxidative stress leading to changes in the redox state of albumin has widely varied effects on its physiological function.